DeClogger® for Enteral Feeding Tubes
Clear obstructed G, J and PEG tubes in less than two minutes.
Clear obstructed G, J and PEG tubes in less than two minutes.
The DeClogger system minimizes the need for procedures and their associated risks, ultimately preventing unnecessary tube replacements while eliminating interruptions for feeding and medication delivery.
DeClogger easily restores nutrition and medication flow in clogged feeding tubes. This safe, flexible device quickly breaks through blockages, reducing premature tube replacement, ensuring timely delivery of essential nutrients.
On FSS Contract and are available through our SDVOSB Distributor.
Made in the U.S.A.
SKU: 911
Package: 10 / Box
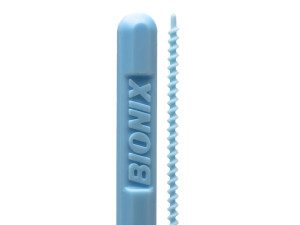
SKU: 912
Package: 10 / Box

SKU: 913
Package: 10 / Box

SKU: 921
Package: 10 / Box

SKU: 922
Package: 10 / Box

SKU: 935
Package: 5 / Box

The DeClogger is indicated for use only in clearing occlusions and/or clogs in feeding tubes (G, J, and/or PEG style) in sizes from 14 Fr – 22 Fr. The DeClogger styles are indicated for use as follows: • DG-00911: For G or J type enteral feeding tubes that are 14 – 16 French, and of a length greater than 39.5 cm (~15.5 in) • DG-00912: For G or J type enteral feeding tubes that are 16 – 22 French, and of a length greater than 39.5 cm (~15.5 in) • DG-00913: For G or J type enteral feeding tubes that are 20 – 22 French, and of a length greater than 39.5 cm (~15.5 in) • DG-00921: For PEG or Replacement type enteral feeding tubes that are 14 – 16 French, and of a length greater than 21.5 cm (~8.5 in) • DG-00922: For PEG or Replacement type enteral feeding tubes that are 18 – 24 French, and of a length greater than 21.5 cm (~8.5 in)
There are no absolute contraindications related to the use of the DeCloggers in clearing enteral feeding tube obstructions.
Professional Use Only
You can order by contacting Bionix directly ([email protected] or 800.551.7096) or contacting your distributor of choice.
The DeClogger® is designed for single-use only.
No, these tubes are longer in length and have a smaller French size.
No, the Declogger® can only be used by a licensed Medical Professional.
The DeClogger is filed under HCPCS Level II Code B9998; this is a National level Medicare Part B code, therefore, it is only billable to DMERC when used on a patient. Since this product is filed under Parenteral and Enteral Nutrition (PEN), coverage is extended to patients in nursing homes and some intermediate and extended care facilities, as well as home care. Requirements for coverage under B9998 include:
The patient must be considered permanently impaired with regard to the need for enteral therapy (CIM65-10 to CIM65-10.3).
There must be a physician’s order for enteral therapy. This will cover all necessary accessories and supplies. This will also cover repairs necessary to make the item serviceable.
There must be a PEN Certificate of Medical Necessity (CMN) accompanying the initial claim submitted and updated every 3 months thereafter. The CMN for PEN covers use of DeClogger.
Since B9998 is a general/miscellaneous code, coverage is NOT guaranteed. Reimbursements for general codes like B9998 are handled on a case-by-case basis by the local DMERC. The best a facility can do to insure reimbursement is to include the required written description of the product and why it was needed. It is important that the facility be as specific as possible when submitting the claim to Medicare and references the DeClogger by name. Include additional information such as what was clogging the tube and why the DeClogger specifically was needed. Including information on clinical effectiveness and cost justifications will also help the claim to be covered.
Please consult with your billing specialist with any further questions.
The DeClogger® has a small disc at the handle that prevents over-insertion. The design of the device with its standardized length and flexible plastic molding are added safeguards.
Suture removal is a critical step in the healing process after surgical procedures or wound closure.
Read More
In a hospital setting, electrical outlets are essential for powering the critical devices and equipment that support patient care.
Read More