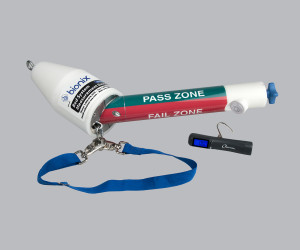
B4000 Bed System Measurement Device
Avoid immediate jeopardy with the B4000. The ONLY device that validates compliance set forth by the FDA.
Avoid immediate jeopardy with the B4000. The ONLY device that validates compliance set forth by the FDA.
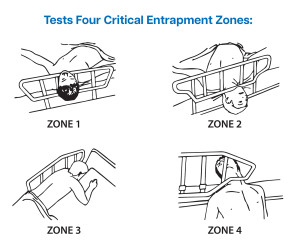
The B4000 Bed System Measurement Device provides clear pass/fail indicators when assessing the four critical entrapment zones of a hospital bed system.
In 2006, the Hospital Bed Safety Workgroup (HBSW) established guidelines to improve bed system safety. From that, the B4000 Bed System Measurement Device was developed, which tests four key entrapment zones to reduce risks.
The B4000 ensures CMS compliance with fast validation, clear pass/fail results and documentable outcomes. It tests the bed rail; rail and mattress; mattress and rail; and compressed mattress and rail end zones.
On FSS Contract and are available through our SDVOSB Distributor.
Made in the U.S.A.
SKU: B4000
Package: 1/EA
1
Professional Use Only
1 Year
You can order by contacting Bionix directly ([email protected] or 800.551.7096) or contacting your distributor of choice.
The FDA guidance document titled “Hospital Bed System Dimensional and Assessment Guidance to Reduce Entrapment” describes the seven entrapment zones of a hospital bed system. The B4000 Bed System Measurement Device was designed to test the four critical entrapment zones. Currently, there is no tool or specifications to test Zones 5-7.
According to the FDA guidance document, the head, neck and chest are at risk for entrapment.
You should be testing to manufacturer’s recommendations, when hardware is replaced, when the mattress is changed, and anytime a new patient is put into the bed. You should discuss the best practices with your risk assessment team.
Yes, each time a rail or mattress is replaced it creates a new bed system. You must re-test a bed system to ensure there is no risk for entrapment.
Yes, if you provide assistive devices as bed attachments for patients, your bed must be tested. In addition, rails and assistive devices often play an important role in a resident’s life. Physical Therapists (PT) often requests these devices to help residents perform their Activity of Daily Living (ADL’s), for independence, rehabilitation and dignity. They often play a vital role in the resident’s daily life as well as individualized quality of care and quality of life needs.
For ease of mattress movement and measurement, the patient should not be in the bed during test procedures.
A bed system must be measured in both the fully raised position and the intermediate position.
Yes, articulate the bed until you see the largest gap, then test the bed system.
The sheets should be on the bed, but pillows and blankets should be removed prior to assessing a bed system.
The strap is a safety harness. Use the strap to secure the cone to the rail being tested. For good safety practices always use the strap to prevent injury to the tester if the tool were to fall while assessing a bed system.
The cone is used to test Zones 1-3, the cone and cylinder are assembled together to test Zone 4.
The scale is used when testing Zone 1 and Zone 2.
No, the tests can be conducted in any order. Please ensure all zones are tested for each bed system.
The bed system must pass all four zones, in both the fully raised position and the intermediate position to “pass”.
The result would be a FAIL for Zone 4. Always ere on the side of caution. If any result is a “close call” the bed fails.
No, the B4000 was not designed to test air fluidized therapy beds, bariatric (obesity) beds, pediatric beds, cribs, or stretchers.
Yes, all results must be documented. CMS (Centers for Medicare & Medicaid Services) wants to see documentation. If it’s not documented, then there’s no proof the testing was completed.
To avoid cross contamination, disinfect the B4000 each time a different bed is assessed. Use only NON-chlorine based disinfection solution(s)/wipe(s) on the tool. Follow the disinfectant solution/wipe manufacturer’s instruction for use. Do NOT immerse or saturate the B4000 or any of its components in disinfecting solution. Ensure the tool is dry before beginning a test.
The B4000 should be shipped back to the technical services office for annual calibration. The calibration service options and instructions can be found on the website by scrolling down to “available accessories” and then the “resources” section on that page or by calling 800-678-7074.
The bed system, as described by the FDA guidance document, is comprised of the bed, rails, and mattress.
Suture removal is a critical step in the healing process after surgical procedures or wound closure.
Read More
In a hospital setting, electrical outlets are essential for powering the critical devices and equipment that support patient care.
Read More